During the project, and starting in December 2016, several tests were performed with injection of an argon-oxygen mixture with 8% by volume of this last gas.
The intended reaction is:
C + ½ O2 —> CO
Whose chemical equilibrium constant is expressed by
K = P CO / (a C x P O2 1 / 2)
On what:
P CO – carbon monoxide partial pressure
a C – chemical activity of carbon in liquid steel. It may be expressed as a function of composition as follows:
ln a C = ln% C x fC +% ix eiC where fC is a known constant at 1600 ° C, % i is the content of each of the other elements present in steel except iron and eiC is the so-called interaction coefficient of that element about carbon.
P O2 is the partial pressure of oxygen.
From this balance one concludes:
- the lower the carbon %, the greater the oxygen partial pressure needed to decarburize for the same carbon monoxide partial pressure;
- the lower the carbon monoxide partial pressure, the lower the equilibrium carbon content for the same oxygen partial pressure, thus favoring decarburization.
On the other hand, very reactive elements coexist in stainless steel, such as:
- chromium, present in the order of 12 to 20%, in the steels manufactured by Ferespe;
- niobium, sometimes residual (in the order of 0,03% or less);
- vanadium, sometimes residual (in the order of 0,05% or less);
- aluminum, always residual (in the order of not more than 0,1%) resulting from the deoxidation process.
It is therefore important, in the process of decarburising steel (in the form of an argon-oxygen mixture), to ensure a partial pressure of oxygen that promotes the reaction, without leading to oxidation of the remaining reactive elements. This is achieved by diluting oxygen with an inert gas (argon in the present case). Thus, although the partial pressure of oxygen is lowered, the carbon monoxide pressure is also lowered. However, as in the equilibrium described above, the partial pressure of oxygen is affected by an exponent ½, whereas carbon monoxide is not, this dilution factor favors the decarburization. Limiting, on the other hand, the oxidation of other elements present in the stainless steel bath.
From thermodynamic calculations that have been performed, it is concluded that:
- for 18% of chromium, and without oxidizing this element, it is theoretically possible to decarburate till a content of 0,03% C with an 8% by volume Ar-O 2 mixture of the latter;
- below this value, the decarburization reaction occurs simultaneously with chromium oxidation, a situation which could only be avoided by lower O2 contents in the mixture or by working under vacuum;
- The presence of levels higher than 0,002% Aluminum, 0,01% Niobium or 0,05% Vanadium implies that oxidation of these elements must occur before decarburization. This represents a loss of process efficiency, namely in terms of the kinetics of the decarburizing reaction.
In the first tests it was therefore intended to test the insufflation of 8% by volume Ar-O2 mixture in steels with low Al, Nb and V contents.
The tests were as follows:
- pressure controlled inflation at the time and adjusted to the progress of the test, in particular in the event of excessive bubbling and projection hazards;
- insufflation time between 1 minute and 3 minutes;
- sampling for chemical analysis immediately before the start of insufflation;
- sampling for chemical analysis immediately after the end of inflation.
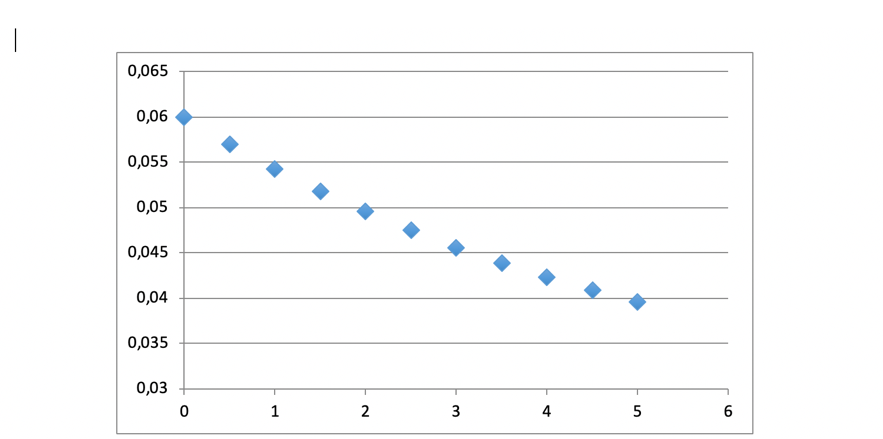
More than 30 tests were made, allowing to describe the decarburizing process by a kinetic model, presented in the following figure.